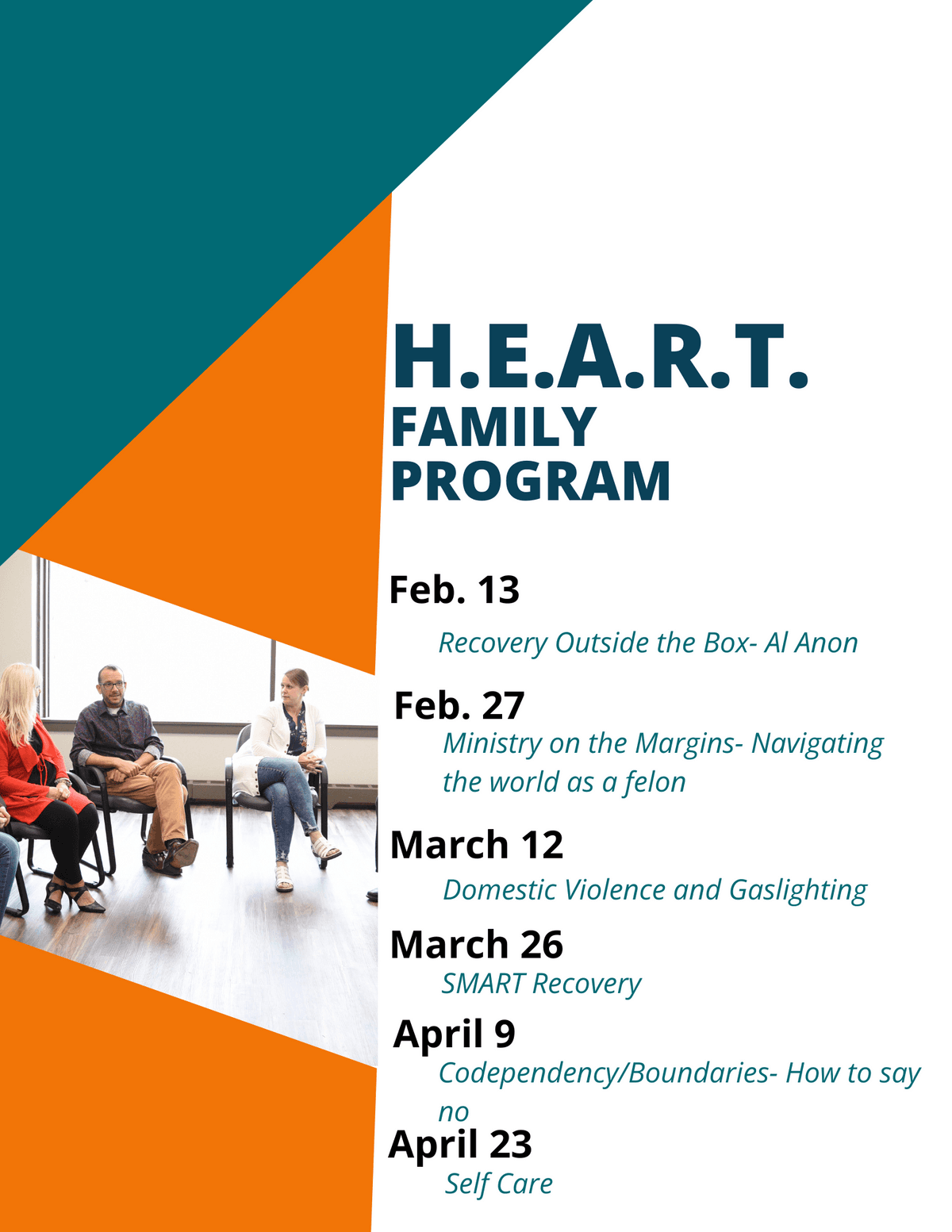
A New Life
Heartview Foundation's mission is to be the provider of choice for quality treatment and education for substance use disorders. Established in 1964, we have treated over 30,000 patients and their families. Patients have come from all over the U.S. and Canada to receive the high-quality care Heartview offers.
-
Fundraisers and events help us raise awareness and generate crucial resources to serve our community. Join us at our next event and see how you can get involved.
Find out more. -
Heartview is constantly expanding to meet the need in our community. Join a fun, impactful team.
Read more.

-
People We've Helped
31,700
-
Intake Calls Last Year
1,907
-
Employees
160+
-
Americans with a Substance Abuse Disorder
15%
Established in 1964, Heartview Foundation has had a significant impact on the lives of many in the community and state.
News & Blog
Not sure if you or a loved one need help with alcohol? Find out what's okay and what's not. Discover the signs of alcohol problems and why it's important to get help. Get the facts and stay safe.
We're excited to welcome you back to the Recovery Chronicles, where we're embarking on an exciting new chapter. We’re thrilled to introduce our new host, Amanda, an LAC at Heartview. In this podcast, we dive deep into the inspiring journeys of individuals who have triumphed over adversity on the path to recovery from drugs and alcohol. Recovery Chronicles can be found at 102.5, Spreaker, or wherever you listen to your favorite podcasts (Apple podcasts, Google podcasts, Spotify, etc).
It has been a year and a half since Emi shared her story with Heartview. Here is her success story 21 months after graduating from Heartview Cando.
-
Heartview has saved my life. My life has changed so much since I’ve been in treatment. Now my number one goal is to stay sober.